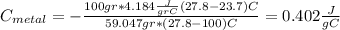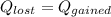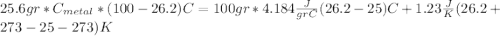For the following scenarios what is the metal? a piece of metal weighing 59.047 g was heated to 100.0 degree c and then put it into 100.0 ml of water (initially at 23.7 degree c). the metal and water were allowed to come to an equilibrium temperature, determined to be 27.8 degree c. assuming no heat lost to the environment, calculate the specific heat of the metal. a 25.6 g piece of metal was taken from a beaker of boiling water at 100.0 degree c and placed directly into a calorimeter holding 100.0 ml of water at 25.0 degree c. the calorimeter heat capacity is 1.23 j/k. given that the final temperature at thermal equilibrium is 26.2 degree c, determine the specific heat capacity of the metal.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mayamabjishovrvq9
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 21.06.2019 22:00, smhrosepetals
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1
Do you know the correct answer?
For the following scenarios what is the metal? a piece of metal weighing 59.047 g was heated to 100...
Questions in other subjects:



Mathematics, 01.07.2021 22:40


Mathematics, 01.07.2021 22:50



Mathematics, 01.07.2021 22:50

Mathematics, 01.07.2021 22:50

Mathematics, 01.07.2021 22:50


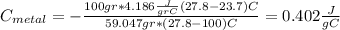
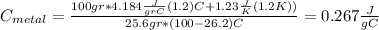
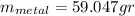 the mass of the metal
the mass of the metal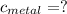 Is the value that we need to find
Is the value that we need to find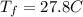 represent the final temperature of equilibrium for the metal and the water
represent the final temperature of equilibrium for the metal and the water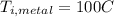 represent the initial temperature for the metal
represent the initial temperature for the metal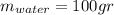 since the density is 1g/ml
since the density is 1g/ml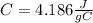 the specific heat for the liquid water
the specific heat for the liquid water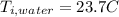 the initial temperature for the water
the initial temperature for the water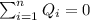 if we have balance then we have this:
if we have balance then we have this: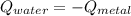
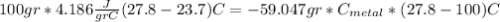
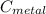 we got:
we got: