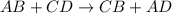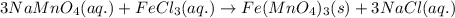Sodium permanganate and iron(iii) chloride are both dissolved in a beaker of water. in a double displacement reaction, two new products are formed. a. write the complete balanced chemical equation (using chemical formulas, not words) for this reaction. include the physical states of the reactants and the products

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1


Chemistry, 23.06.2019 00:00, PlzNoToxicBan
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1
Do you know the correct answer?
Sodium permanganate and iron(iii) chloride are both dissolved in a beaker of water. in a double disp...
Questions in other subjects:

Mathematics, 28.08.2019 20:40

History, 28.08.2019 20:40

Social Studies, 28.08.2019 20:40


History, 28.08.2019 20:40


Biology, 28.08.2019 20:40

History, 28.08.2019 20:40


History, 28.08.2019 20:40