Chemistry, 24.01.2020 05:31, scarlettp13
Zinc metal reacts with silver nitrate according to the reaction:
zn(s) + 2agno3(aq)zn(no3 )2 (aq) + 2ag(s)
calculate the mass of ag that forms when 3.00g of zinc metal is placed in an aqueous solution containing 3.75g of silver nitrate?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
Do you know the correct answer?
Zinc metal reacts with silver nitrate according to the reaction:
zn(s) + 2agno3(aq)zn(n...
zn(s) + 2agno3(aq)zn(n...
Questions in other subjects:


Biology, 15.07.2019 17:00

History, 15.07.2019 17:00

Mathematics, 15.07.2019 17:00



Computers and Technology, 15.07.2019 17:00



Arts, 15.07.2019 17:00


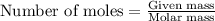 ....(1)
....(1)
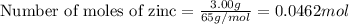
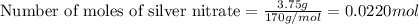
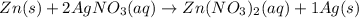
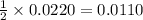 moles of zinc
moles of zinc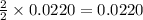 moles of silver
moles of silver