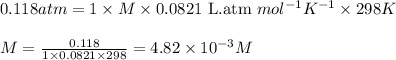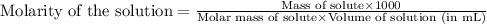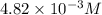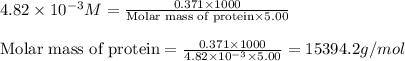Chemistry, 24.01.2020 01:31, niyyyareligion
371. mg of an unknown protein are dissolved in enough solvent to make 5.00 ml of solution. the osmotic pressure of this solution is measured to be at 0.118 atm at 25 c
calculate the molar mass of the protein.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3


Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
Do you know the correct answer?
371. mg of an unknown protein are dissolved in enough solvent to make 5.00 ml of solution. the osmot...
Questions in other subjects:



Social Studies, 12.02.2020 22:46



History, 12.02.2020 22:47

Mathematics, 12.02.2020 22:47


Chemistry, 12.02.2020 22:47


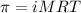
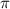 = osmotic pressure of the solution = 0.118 atm
= osmotic pressure of the solution = 0.118 atm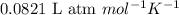
![25^oC=[273+25]=298K](/tpl/images/0468/1226/6a9f9.png)