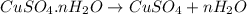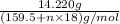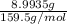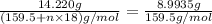Copper(ii) sulfate forms a bright blue hydrate with the formula cuso 4 ⋅ n h 2 o ( s ) . if this hydrate is heated to a high enough temperature, h 2 o ( g ) can be driven off, leaving the grey‑white anhydrous salt cuso 4 ( s ) . a 14.220 g sample of the hydrate was heated to 300 ∘ c . the resulting cuso 4 ( s ) had a mass of 8.9935 g . calculate the val

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, leilanimontes714
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 02:10, apowers6361
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 05:30, saleenhernandez83
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1
Do you know the correct answer?
Copper(ii) sulfate forms a bright blue hydrate with the formula cuso 4 ⋅ n h 2 o ( s ) . if this hyd...
Questions in other subjects:

Mathematics, 09.01.2020 16:31

Chemistry, 09.01.2020 16:31

Mathematics, 09.01.2020 16:31


Mathematics, 09.01.2020 16:31



Chemistry, 09.01.2020 16:31

Mathematics, 09.01.2020 16:31


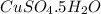 .
.