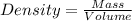To save time you can approximate the initial volume of water to ±1 ml and the initial mass of the solid to ±1 g. for example, if you are asked to add 23 ml of water, add between 22 ml and 24 ml. which metals in each of the following sets will have equal density?
1. 20.2 g gold placed in 21.6 ml of water and 12.0 g copper placed in 21.6 ml of water.
2. 20.2 g silver placed in 21.6 ml of water and 12.0 g silver placed in 21.6 ml of water.
3. 15.2 g copper placed in 21.6 ml of water and 50.0 g copper placed in 23.4 ml of water.
4. 15.4 g gold placed in 20.0 ml of water and 15.7 g silver placed in 20.0 ml of water.
5. 20.2 g silver placed in 21.6 ml of water and 20.2 g copper placed in 21.6 ml of water.
6. 11.2 g gold placed in 21.6 ml of water and 14.9 g gold placed in 23.4 ml of water.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Do you know the correct answer?
To save time you can approximate the initial volume of water to ±1 ml and the initial mass of the so...
Questions in other subjects:



History, 25.07.2019 08:00


Health, 25.07.2019 08:00

History, 25.07.2019 08:00

Mathematics, 25.07.2019 08:00