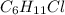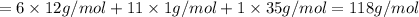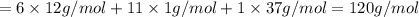Halogenated compounds are particularly easy to identify by their mass spectra because chlorine and bromine occur naturally as mixtures of two abundant isotopes. chlorine occurs as 35cl (75.8%) and 37cl (24.2%); bromine occurs as 79br (50.7%) and 81br (49.3%). for the compound chlorocyclohexane, c6h11cl: at what masses do the molecular ions occur? (list in order of increasing mass separated by commas, e. g. 120,122.) what are the percentages of each molecular ion?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, jalenshayewilliams
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 18:30, ashleymer384
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 23.06.2019 01:00, tjeffers90028
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 02:00, hermesrobles
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
Do you know the correct answer?
Halogenated compounds are particularly easy to identify by their mass spectra because chlorine and b...
Questions in other subjects:


Biology, 28.08.2019 09:30




Chemistry, 28.08.2019 09:30

Social Studies, 28.08.2019 09:30

Spanish, 28.08.2019 09:30


Mathematics, 28.08.2019 09:30