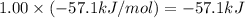Hcl(aq)+naoh(aq)→nacl(aq)+h2o(l)δh° =−57.1kj/molrxn
the chemical equation above represents th...

Chemistry, 23.01.2020 20:31, powellkolbie
Hcl(aq)+naoh(aq)→nacl(aq)+h2o(l)δh° =−57.1kj/molrxn
the chemical equation above represents the reaction between hcl(aq) and naoh(aq). when equal volumes of 1.00mhcl(aq) and 1.00mnaoh(aq) are mixed, 57.1kj of heat is released. if the experiment is repeated with 2.00mhcl(aq), how much heat would be released?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, akatsionis25
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 21.06.2019 22:00, creepycrepes
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 21.06.2019 22:30, Cnolteb5663
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b. slope c. benchmark d. index contour
Answers: 1

Do you know the correct answer?
Questions in other subjects:



Mathematics, 25.12.2020 01:50

Chemistry, 25.12.2020 01:50

Mathematics, 25.12.2020 01:50


Biology, 25.12.2020 01:50

Mathematics, 25.12.2020 01:50


Physics, 25.12.2020 01:50


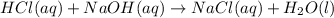 ΔH°=-57.1kJ/mol
ΔH°=-57.1kJ/mol of NaOH
of NaOH