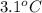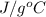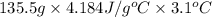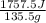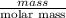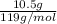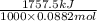Acoffee cup calorimeter initially contains 125g of water at 24.2oc. 10.5g of potassium bromide also at 24.2oc is added to the water. after the kbr dissolves the final temperature is 21.1oc. calculate the enthalpy change for dissolving the salt in j/g and kj/mol. assume specific heat of solution is 4.18j/goc.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 08:40, mathisaqeosmw
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3
Do you know the correct answer?
Acoffee cup calorimeter initially contains 125g of water at 24.2oc. 10.5g of potassium bromide also...
Questions in other subjects:

History, 20.08.2019 23:30

Mathematics, 20.08.2019 23:30

Mathematics, 20.08.2019 23:30


Mathematics, 20.08.2019 23:30




Mathematics, 20.08.2019 23:30