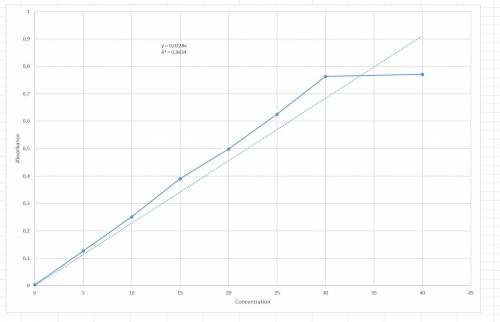
Chemistry, 23.01.2020 05:31, lraesingleton
The following results were obtained when each of a series of standard silver solutions was analyzed by flame-atomic absorption spectrometry: concentration on 0 5 10 15 20 25 30 40 (ng. ml1) absorbance(r. u.) 0.003 0.127 0.251 0.390 0.498 0.625 0.763 0.771 determine the slope and intercept of the calibration plot, along with their confidence limits (95%). using the data from exercise 1 above, estimate the confidence limits for the silver concentration in: a) a sample giving an absorbance of 0.456 in a single determination. b) a sample giving absorbance values of 0.308, 0.317, 0.347, and 0.412 in four separate determinations. graph: a straight line excel plot is shown in the question

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 13:30, princessroseee769
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Do you know the correct answer?
The following results were obtained when each of a series of standard silver solutions was analyzed...
Questions in other subjects:



Mathematics, 22.07.2019 15:00


Mathematics, 22.07.2019 15:00

English, 22.07.2019 15:00



Biology, 22.07.2019 15:00