Fe(s) + 2hcl(aq) --> fecl2(aq) + h2(g)
when a student adds 30.0 ml of 1.00 m hcl to...

Chemistry, 23.01.2020 03:31, coollid876
Fe(s) + 2hcl(aq) --> fecl2(aq) + h2(g)
when a student adds 30.0 ml of 1.00 m hcl to 0.56 g of powdered fe, a reaction occurs according to the equation above. when the reaction is complete at 273 k and 1.0 atm, which of the following is true?
a) hcl is in excess, and 0.100 mol of hcl remains unreacted.
d) 0.22 l of h2 has been produced.
the correct answer is d. i can't figure out why a is wrong.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, wkalpakchi
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1


Chemistry, 23.06.2019 12:50, vinniemccray70
What is the daughter nucleus produced a. when 217(at) undergoes alpha decay? b. when 103(mo) undergoes beta decay? c. when 188(hg) undergoes positron emission?
Answers: 1

Chemistry, 23.06.2019 14:00, teamsadie8426
Which statement describes the arrhenius interpretation of acids and bases?
Answers: 1
Do you know the correct answer?
Questions in other subjects:

English, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

French, 13.09.2020 20:01

History, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01

Mathematics, 13.09.2020 20:01


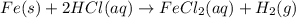
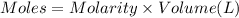
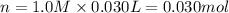
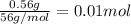
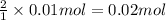 of HCl
of HCl



