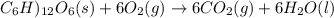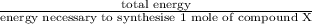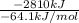Chemistry, 23.01.2020 03:31, coleman310
Assume that the complete combustion of one mole of fructose, a monosaccharide, to carbon dioxide and water liberates 2810 kj (δg°\' = –2810 kj/mol). if the energy generated by the combustion of fructose is entirely converted to the synthesis of a hypothetical compound x, calculate the number of moles of the compound that could theoretically be generated. use the value δg°\'compound x = − 64.1 kj/mol kj/mol. round your answer to two significant figures.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:00, penelopymorales24
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 08:40, alexisbcatlett14
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1
Do you know the correct answer?
Assume that the complete combustion of one mole of fructose, a monosaccharide, to carbon dioxide and...
Questions in other subjects:

Mathematics, 24.06.2019 16:30

History, 24.06.2019 16:30






English, 24.06.2019 16:30


Mathematics, 24.06.2019 16:30