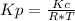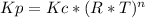Chemistry, 23.01.2020 03:31, bakaoffire
Consider the following chemical equilibrium: c(s) +2h2 (g) ch4 (g) now write an equation below that shows how to calculate from for this reaction at an absolute temperature . you can assume is comfortably above room temperature. if you include any common physical constants in your equation be sure you use their standard symbols, found in the aleks calculator.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, misspicafunpoke
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2


Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
Do you know the correct answer?
Consider the following chemical equilibrium: c(s) +2h2 (g) ch4 (g) now write an equation below that...
Questions in other subjects:

Mathematics, 15.12.2020 22:10

Mathematics, 15.12.2020 22:10

English, 15.12.2020 22:10

Mathematics, 15.12.2020 22:10

Health, 15.12.2020 22:10


Arts, 15.12.2020 22:10



Social Studies, 15.12.2020 22:10