
Chemistry, 22.01.2020 23:31, jjjjjjjbvhnchjkj
Assume that all samples listed below have the same pressure and temperature. which would have the greatest volume? a.1 gram of o2 b. all have the same volume c.1 gram of ar d.1 gram of h2 e. not enough information

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 00:00, vanessacox45
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
Do you know the correct answer?
Assume that all samples listed below have the same pressure and temperature. which would have the gr...
Questions in other subjects:




English, 15.11.2020 23:10

Mathematics, 15.11.2020 23:10


Advanced Placement (AP), 15.11.2020 23:10



History, 15.11.2020 23:10


 .
.  (At constant temperature and pressure)
(At constant temperature and pressure) 
 = initial volume of gas
= initial volume of gas 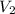 = final volume of gas
= final volume of gas  = initial number of moles
= initial number of moles  = final number of moles
= final number of moles 







