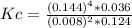Chemistry, 22.01.2020 22:31, Shamplo8817
Steam reforming of methane ( ch4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 125l tank with 20 mol of methane gas and 10 mol of water vapor at 38 degrees celsius. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of hydrogen gas to be 18 mol . calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, robert7248
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 11:00, RidhaH
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural. question 2 reflects a moral or social value. question 3 refers to something that can be measured. question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 12:00, sophiaa23
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2
Do you know the correct answer?
Steam reforming of methane ( ch4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hy...
Questions in other subjects:

Mathematics, 19.01.2021 18:10

Social Studies, 19.01.2021 18:10

English, 19.01.2021 18:10

Mathematics, 19.01.2021 18:10


Mathematics, 19.01.2021 18:10


Mathematics, 19.01.2021 18:10

Mathematics, 19.01.2021 18:10

SAT, 19.01.2021 18:10


![Kc = \frac{[H_2]^4*[CO_2]}{[H_2O]^2*[CH_4]}](/tpl/images/0466/2862/a2afc.png)