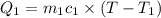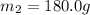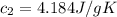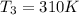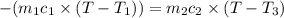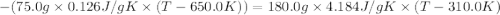Chemistry, 22.01.2020 20:31, justin5163
A75.0 g piece of gold at 650. k is dropped into 180. g of h2o(l) at 310. k in an insulated container at 1 bar pres- sure. calculate the temperature of the system once equilib- rium has been reached. assume that cp, m for au and h2o is constant at their values for 298 k throughout the temperature range of interest.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
Do you know the correct answer?
A75.0 g piece of gold at 650. k is dropped into 180. g of h2o(l) at 310. k in an insulated container...
Questions in other subjects:


Mathematics, 06.02.2021 17:40


Mathematics, 06.02.2021 17:40




Geography, 06.02.2021 17:40



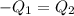
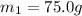
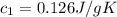
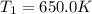
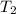 =T
=T