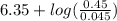Chemistry, 22.01.2020 06:32, itz0nlyheav
Which of the following has the strongest buffering capacity? a. h2o b. 0.1 m hcl c. 0.1 m carbonic/bicarbonate (h2co3/hco3-) at ph ~pka d. 0.2 m carbonic/bicarbonate (h2co3/hco3-) at ph ~pka

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, gonzalesalexiaouv1bg
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1


Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3
Do you know the correct answer?
Which of the following has the strongest buffering capacity? a. h2o b. 0.1 m hcl c. 0.1 m carbonic/...
Questions in other subjects:

Health, 03.11.2020 16:40



Mathematics, 03.11.2020 16:40







 ) and Sodium bicarbonate (
) and Sodium bicarbonate ( ) forms an acidic buffer.
) forms an acidic buffer.
![pK_{a} + log(\frac{[Salt]}{[Acid]})](/tpl/images/0465/3354/fdbea.png) ........... (1)
........... (1)
![\frac{[Salt]}{[Acid]}](/tpl/images/0465/3354/6743a.png) = 1
.
= 1
. ![log (\frac{[Salt]}{[Acid]})](/tpl/images/0465/3354/555b1.png) = 0
= 0
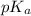 (when acid and salt are equal in concentration)
(when acid and salt are equal in concentration)
![[NaHCO_{3}]](/tpl/images/0465/3354/aa52d.png) = 0.045 M and,
= 0.045 M and, ![[H_{2}CO_{3}]](/tpl/images/0465/3354/77e8d.png) = 0.45 M
= 0.45 M
![6.35 + log([NaHCO_{3}][[H_{2}CO_{3}])](/tpl/images/0465/3354/ada81.png)
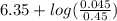
![6.35 + log(\frac{[NaHCO_{3}]}{[H_{2}CO_{3}]})](/tpl/images/0465/3354/06a47.png)