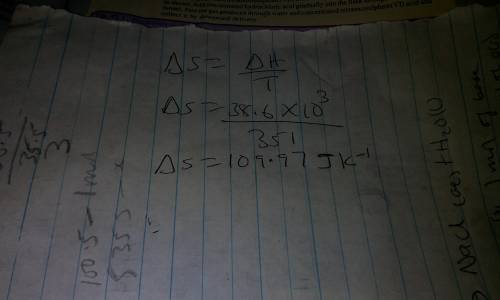Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 23.06.2019 04:20, tyrickdavis1
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1

Do you know the correct answer?
The boiling point of ethanol is 78 °c. the molar heat(i. e., enthalpy)of vaporization of ethanol is...
Questions in other subjects:


English, 22.11.2020 08:00






Mathematics, 22.11.2020 08:00

History, 22.11.2020 08:00