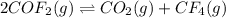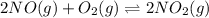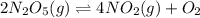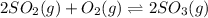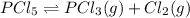Chemistry, 22.01.2020 05:31, clangclang8732
Predict the shift in equilibrium position that will occur for each of the following reactions when the volume of the reaction container is increased.
a) 2cof2(g)⇌co2(g)+cf4(g).
i) to the left.
ii) to the right.
iii) does not shift.
b) 2no(g)+o2(g)⇌2no2(g).
i) to the left.
ii) to the right.
iii) does not shift.
c) 2n2o5(s)⇌4no2(g)+o2(g).
i) to the left.
ii) to the right.
iii) does not shift.
d) 2so2(g)+o2(g)⇌2so3(g).
i) to the left.
ii) to the right.
iii) does not shift.
e) pcl5(g)⇌pcl3(g)+cl2(g).
i) to the left.
ii) to the right.
iii) does not shift.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 23.06.2019 00:30, coralaguilar1702
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 04:00, josephicarusmarrujo
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1
Do you know the correct answer?
Predict the shift in equilibrium position that will occur for each of the following reactions when t...
Questions in other subjects:

Mathematics, 19.01.2020 22:31

Mathematics, 19.01.2020 22:31


Mathematics, 19.01.2020 22:31




Physics, 19.01.2020 22:31

History, 19.01.2020 22:31