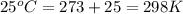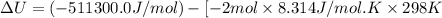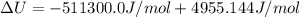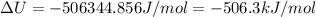Chemistry, 22.01.2020 04:31, george6871
The standard internal energy change for a reaction can be symbolized as δ u ∘ rxn or δ e ∘ rxn . for each reaction equation, calculate the energy change of the reaction at 25 ∘ c and 1.00 bar . sn ( s ) + 2 cl 2 ( g ) ⟶ sncl 4 ( l ) δ h ∘ rxn = − 511.3 kj/mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 10:30, Brookwiggington8814
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1


Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
Do you know the correct answer?
The standard internal energy change for a reaction can be symbolized as δ u ∘ rxn or δ e ∘ rxn . for...
Questions in other subjects:

Mathematics, 03.01.2021 02:40





Social Studies, 03.01.2021 02:40

English, 03.01.2021 02:40


English, 03.01.2021 02:40

Mathematics, 03.01.2021 02:40


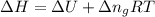
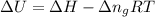
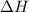 = change in enthalpy =
= change in enthalpy = 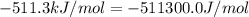
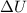 = change in internal energy = ?
= change in internal energy = ?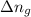 = change in moles
= change in moles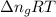 = 0
= 0