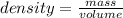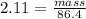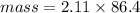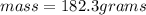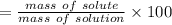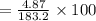Chemistry, 22.01.2020 04:31, ayoismeisjjjjuan
Asolution is made by dissolving 4.87 g of potassium nitrate in water to a final volume of 86.4 ml solution. what is the weight/weight % or percent by mass of the solute? (i got 5.64% but it said it was incorrect, and i can't figure it out? )

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, fbillinton
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 17:00, davisnaziyahovz5sk
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
Do you know the correct answer?
Asolution is made by dissolving 4.87 g of potassium nitrate in water to a final volume of 86.4 ml so...
Questions in other subjects:



Social Studies, 28.07.2019 22:30

Mathematics, 28.07.2019 22:30

Arts, 28.07.2019 22:30


Mathematics, 28.07.2019 22:30

Arts, 28.07.2019 22:30

History, 28.07.2019 22:30

Arts, 28.07.2019 22:30