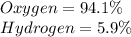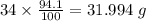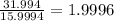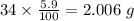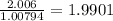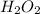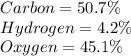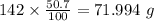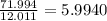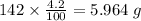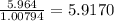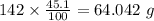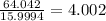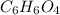Determine the molecular formula for each compound.
a) 94.1% oxygen and 5.9% hydrogen; molar mass = 34g
b) 50.7% carbon, 4.2% hydrogen, and 45.1% oxygen; molar mass = 142g
(would greatly appreciated if someone could explain the process, also use the correct amount of significant digits)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1


Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1
Do you know the correct answer?
Determine the molecular formula for each compound.
a) 94.1% oxygen and 5.9% hydrogen; molar...
a) 94.1% oxygen and 5.9% hydrogen; molar...
Questions in other subjects:

English, 30.06.2019 21:00


Spanish, 30.06.2019 21:00


Mathematics, 30.06.2019 21:00


Mathematics, 30.06.2019 21:00


History, 30.06.2019 21:00


 molecules.
molecules.