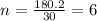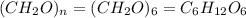Chemistry, 21.01.2020 23:31, Spoiledgirl2905
The empirical formula for a compound is ch2o, and the molar mass is 180.2 g/mol. which is the molecular formula for this compound? a) c6h12o6
b) c7h16o5
c) c8h20o4
d) c3h6o3

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, blondieb1722
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2


Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
Do you know the correct answer?
The empirical formula for a compound is ch2o, and the molar mass is 180.2 g/mol. which is the molecu...
Questions in other subjects:


Mathematics, 10.12.2020 23:20

Spanish, 10.12.2020 23:20

Mathematics, 10.12.2020 23:20

Biology, 10.12.2020 23:20

English, 10.12.2020 23:20





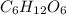
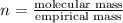
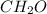 and the molar mass of compound is, 180.2 gram/mol.
and the molar mass of compound is, 180.2 gram/mol.