
A= ε l c. (a) define the terms in the formula: a = ε l c. (pick your answers using the letter of the correct definition.)
a. pathlength of light through the cell
b. concentration, in mol/l, of the stock solution from which the sample was made
c. absorbance measured by the spectrometer
d. molar absorptivity, a constant unique to that substance at that wavelength
e. the wavelength of the light being used for the measurement
f. concentration, in mol/l, of the sample solution being measured definition of l definition of c definition of ε definition of a

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, melidacampos12
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 22:30, COOLIOMARIS
What three things does a balanced equation show you?
Answers: 1

Chemistry, 23.06.2019 02:30, erikacastro259
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
Do you know the correct answer?
A= ε l c. (a) define the terms in the formula: a = ε l c. (pick your answers using the letter of th...
Questions in other subjects:


Mathematics, 07.05.2020 05:05



English, 07.05.2020 05:05



Mathematics, 07.05.2020 05:05



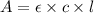
 = molar absorptivity of this solution
= molar absorptivity of this solution




