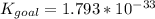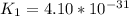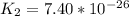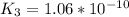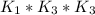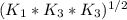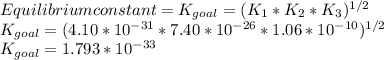Chemistry, 21.01.2020 06:31, joelpimentel
Determine the value of the equilibrium constant, kgoal, for the reactionn2(g)+h2o(g)⇌no(g)+12n2h4(g ), kgoal=? by making use of the following information: 1. n2(g)+o2(g)⇌2no(g), k1 = 4.10×10−312. n2(g)+2h2(g)⇌n2h4(g), k2 = 7.40×10−263. 2h2o(g)⇌2h2(g)+o2(g), k3 = 1.06×10−10express your answer numerically.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, toniawu18
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2


Do you know the correct answer?
Determine the value of the equilibrium constant, kgoal, for the reactionn2(g)+h2o(g)⇌no(g)+12n2h4(g...
Questions in other subjects:


Mathematics, 12.11.2020 20:40

Mathematics, 12.11.2020 20:40


Mathematics, 12.11.2020 20:40

Mathematics, 12.11.2020 20:40


Mathematics, 12.11.2020 20:40

History, 12.11.2020 20:40