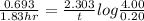Fluorine-18 (18f) is a radioactive isotope use in a variety of medical imaging procedures including positron emission tomography (pet) scans.
fluorine-18 decays by positron emission with a half-life of 1.83 hours.
(1) when 18f undergoes positron emission, the product nucleus is,
a) 18o b) 19ne c) 14n d) 17f
(2) a typical dose of 18f used for a pet scan has an activity of 4.00 millicuries. how long will it take for 95% of the 18f to decay?
a) 1.74 hrs b) 5.49 min c) 8.13 minutes d) 7.91 hrs.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, jude3412
In an effort to address concerns about global warming, a power plant in portland, oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2


Do you know the correct answer?
Fluorine-18 (18f) is a radioactive isotope use in a variety of medical imaging procedures including...
Questions in other subjects:


Mathematics, 22.01.2021 19:20

Health, 22.01.2021 19:20


English, 22.01.2021 19:20


Mathematics, 22.01.2021 19:20




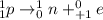
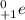 . Therefore, when a positron emission occurs then the resultant nuclei atomic number decreases by a unit mass.
. Therefore, when a positron emission occurs then the resultant nuclei atomic number decreases by a unit mass. 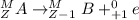
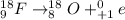
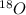 .
.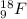 isotope is as follows.
isotope is as follows.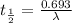
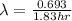
![[N]_{o}](/tpl/images/0463/6241/b711d.png) ) = 4.00 millicuries
) = 4.00 millicuries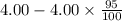
![\lambda = \frac{2.303}{t} log \frac{[N]_{o}}{[N]_{t}}](/tpl/images/0463/6241/b899d.png)