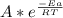Chemistry, 21.01.2020 01:31, cierramcdonald
A)if it takes 0.20 min to decompose 15% of a 0.300 m solution of nitrosyl chloride, what is k(the rate constant)? the decomposition of nitrosyl chloride is a second order reaction: nocl(> no(g)+1/2cl2(g)b)calculate the activation energy? when the temperature increases from 15c to 25c for a certain reaction, its rate constant doubles. calculate the activation energy, ea+ (remember: with r=8.31 j/mol. k, ea must be expressed in j/mol)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 04:40, dd123984
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
Do you know the correct answer?
A)if it takes 0.20 min to decompose 15% of a 0.300 m solution of nitrosyl chloride, what is k(the ra...
Questions in other subjects:


History, 08.10.2020 06:01


Mathematics, 08.10.2020 06:01



Biology, 08.10.2020 06:01