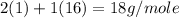Chemistry, 21.01.2020 01:31, victoria1831
Given that the atomic weight of hydrogen is appoximately 1 gram per mole, use the method above to estimate teh average molecular mass for water vapor

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:00, bryn2433
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3


Chemistry, 23.06.2019 06:00, asalimanoucha2v
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3
Do you know the correct answer?
Given that the atomic weight of hydrogen is appoximately 1 gram per mole, use the method above to es...
Questions in other subjects:

Mathematics, 27.04.2021 14:00






English, 27.04.2021 14:00

Social Studies, 27.04.2021 14:00

Mathematics, 27.04.2021 14:00

History, 27.04.2021 14:00


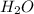 .
.