
Chemistry, 20.01.2020 21:31, maystrenko53
Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. an industrial chemist studying this reaction fills a 500 ml flask with 4.8 atm of ammonia gas and 1.9 atm of oxygen gas at 33 degress celsius. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of nitrogen gas to be 0.63 atm. calculate the pressure equilibrium constant for the combustion of ammonia at the final temperature of the mixture.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:40, wiltseliz4800
What does the process of natural selection involve
Answers: 1

Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2
Do you know the correct answer?
Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its r...
Questions in other subjects:

History, 09.04.2020 20:26

Business, 09.04.2020 20:26

Mathematics, 09.04.2020 20:26





Geography, 09.04.2020 20:26

Mathematics, 09.04.2020 20:26


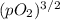 ]
]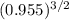 ]
]



