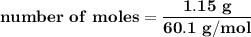Suppose that 1.15 g of rubbing alcohol (c3h8o) evaporates from a 65.0-g aluminum block. if the aluminum block is initially at 25 °c, what is the final temperature of the block after the evapo- ration of the alcohol? assume that the heat required for the vaporization of the alcohol comes only from the aluminum block and that the alcohol vaporizes at 25 °c.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 01:30, ayoismeisalex
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1
Do you know the correct answer?
Suppose that 1.15 g of rubbing alcohol (c3h8o) evaporates from a 65.0-g aluminum block. if the alumi...
Questions in other subjects:




History, 28.01.2020 05:31


Mathematics, 28.01.2020 05:31

Mathematics, 28.01.2020 05:31