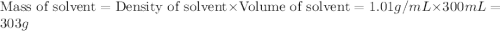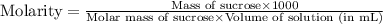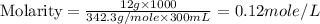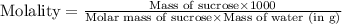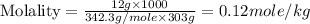Astudent dissolves 12.g of sucrose c12h22o11 in 300.ml of a solvent with a density of 1.01/gml . the student notices that the volume of the solvent does not change when the sucrose dissolves in it. calculate the molarity and molality of the student's solution. round both of your answers to 2 significant digits.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:50, adrian08022
Use the standard enthalpies of formation for the reactants and products to solve for the δhrxn for the following reaction. (the δhf of c2h4 is 52.26 kj/mol, co2 is -393.509 kj/mol, and h2o is -241.818 kj.) c2h4 (g) + 3o2(g) 2co2 (g) + 2h2o(g) δhrxn = the reaction is .
Answers: 3


Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1
Do you know the correct answer?
Astudent dissolves 12.g of sucrose c12h22o11 in 300.ml of a solvent with a density of 1.01/gml . the...
Questions in other subjects:




Chemistry, 26.08.2019 17:10

Mathematics, 26.08.2019 17:10

Computers and Technology, 26.08.2019 17:10

History, 26.08.2019 17:10


English, 26.08.2019 17:10

English, 26.08.2019 17:10