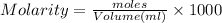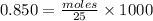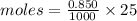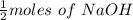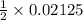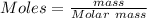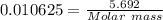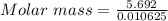Adiprotic acid is composed of carbon, hydrogen and oxygen. it is 60.6% c, and 3.58% h by mass. a titration requires 25 ml of 0.850mnaoh to neutralize 5.692g of the acid. what is the molecular formula for diprotic acid?
a- c27h19012
b- c54h38024
c- c22h20o13
d- c12h15o4
e- c15h12o4

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, andaws21
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 00:00, brookemcelhaney
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
Do you know the correct answer?
Adiprotic acid is composed of carbon, hydrogen and oxygen. it is 60.6% c, and 3.58% h by mass. a tit...
Questions in other subjects:

Mathematics, 06.05.2020 07:15

Mathematics, 06.05.2020 07:15


English, 06.05.2020 07:15


English, 06.05.2020 07:15