
Chemistry, 18.01.2020 05:31, culturedxnat
Classify the following substances as a bronsted-lowry acid, bronsted-lowry base, lewis acid, and/or lewis base.
hcl, bf_3,
ccl_3, -h
ch_2o, ch_3cl
-och_3 , nh_3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Do you know the correct answer?
Classify the following substances as a bronsted-lowry acid, bronsted-lowry base, lewis acid, and/or...
Questions in other subjects:




Mathematics, 25.02.2021 01:00


Mathematics, 25.02.2021 01:00

Chemistry, 25.02.2021 01:00


Mathematics, 25.02.2021 01:00


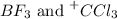 : Lewis-acid
: Lewis-acid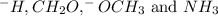 : Bronsted-Lowry base
: Bronsted-Lowry base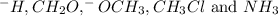 : Lewis-base
: Lewis-base



