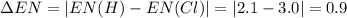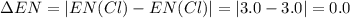Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1


Do you know the correct answer?
Consider the following electronegativity values:
h = 2.1, cl = 3.0, f = 4.0
which mole...
h = 2.1, cl = 3.0, f = 4.0
which mole...
Questions in other subjects:

Geography, 30.09.2019 09:30

Computers and Technology, 30.09.2019 09:30


Business, 30.09.2019 09:30

History, 30.09.2019 09:30

Mathematics, 30.09.2019 09:30

Computers and Technology, 30.09.2019 09:30

Mathematics, 30.09.2019 09:30

Mathematics, 30.09.2019 09:30

History, 30.09.2019 09:30