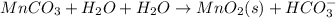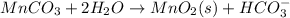Write a half-reaction for the oxidation of the manganese in mnco3(s) to mno2(s) in neutral groundwater where the carbonate-containing species in the product is hco3–(aq). add h2o and h+ to balance the h and o atoms in the equation. do not add electrons; you may leave the half-reaction unbalanced with respect to charge

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
Do you know the correct answer?
Write a half-reaction for the oxidation of the manganese in mnco3(s) to mno2(s) in neutral groundwat...
Questions in other subjects:



Health, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20




Mathematics, 18.03.2021 01:20



 to
to  .:
.: