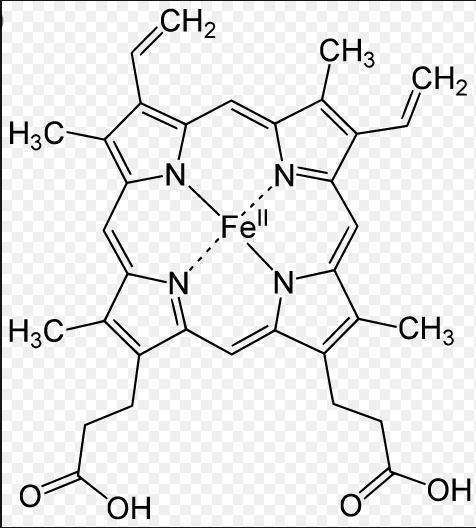
Hemoglobin in your blood does not use elemental iron. it uses iron in the form of fe2+(aq).
iron reacts with acid (represented as h+(aq)) to produce fe2+(aq) and hydrogen gas.
write a balanced chemical reaction, including phase symbols, to describe the reaction of iron with acid.
write a hypothesis that may explain how and where neutral iron is converted to fe2+ cation in the human body.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:40, mandilynn22
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Chemistry, 23.06.2019 05:30, xarianna2007
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1

Chemistry, 23.06.2019 05:40, shelbylynn1093
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
Do you know the correct answer?
Hemoglobin in your blood does not use elemental iron. it uses iron in the form of fe2+(aq).
Questions in other subjects:

English, 04.10.2019 22:00


History, 04.10.2019 22:00

Mathematics, 04.10.2019 22:00



Mathematics, 04.10.2019 22:00




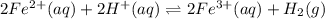
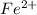 (aq) cation. There will be an oxidation-half reaction and a reduction-half reaction. Equations for this reaction are as follows.
(aq) cation. There will be an oxidation-half reaction and a reduction-half reaction. Equations for this reaction are as follows.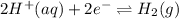 ...... (2)
...... (2)