

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1
Do you know the correct answer?
For h3po4, ka1 = 7.3 x 10^-3, ka2 = 6.2 x 10^-6, and ka3 = 4.8 x 10^-13. a 0.10 m aqueous solution o...
Questions in other subjects:





Mathematics, 05.05.2020 18:59



Mathematics, 05.05.2020 18:59



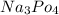 therefore would be "strongly basic."
therefore would be "strongly basic."



