

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, hjamya17
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1


Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 23.06.2019 01:00, shartiarahoward
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
Do you know the correct answer?
Avoltaic cell consists of a hg/hg22+ electrode (e°= 0.85 v) and a sn/sn2+ electrode (e°= –0.14 v). c...
Questions in other subjects:

Mathematics, 17.02.2020 05:18


English, 17.02.2020 05:18



Biology, 17.02.2020 05:19

Mathematics, 17.02.2020 05:19


Health, 17.02.2020 05:19

Mathematics, 17.02.2020 05:19


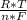 *ln(Q)
*ln(Q)![\frac{[C]^{c}*[D]^{d} }{[A]^{a}*[B]^{b} }](/tpl/images/0460/2641/2f4bf.png) In this case [C] and [D], they refer to the partial pressures, also known as molar concentrations in the case of gases or ions in solution, for the reaction products. [A] and [B] are also partial pressures but in the case of reagents. The exponents refer to the amount of moles that make up each substance that is participating in the reaction. Substances that are in a solid state have a unit concentration (that is, their value is 1), so they do not appear in Q.
In this case [C] and [D], they refer to the partial pressures, also known as molar concentrations in the case of gases or ions in solution, for the reaction products. [A] and [B] are also partial pressures but in the case of reagents. The exponents refer to the amount of moles that make up each substance that is participating in the reaction. Substances that are in a solid state have a unit concentration (that is, their value is 1), so they do not appear in Q.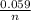 *ln(Q) Equation (A)
*ln(Q) Equation (A)![\frac{[Sn^{2+}] }{[Hg_{2} ^{+2} ]}](/tpl/images/0460/2641/af92b.png) where [Hg₂²⁺] is 0.24 M
where [Hg₂²⁺] is 0.24 M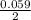 *ln
*ln![\frac{[Sn]^{2+} }{0.24}](/tpl/images/0460/2641/8f497.png)




