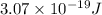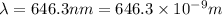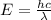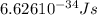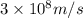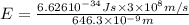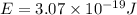When an electron in a 2p orbital of a particular atom makes a transition to the 2s orbital, a photon of approximate wavelength 646.3 nm is emitted. the energy difference between these 2p and 2s orbitals is: . 3.07 ã 10^â28 jb. 3.07 ã 10^â19 jc. 3.07 ã 10^â17 jd. 1.28 ã 10^â31 je. none of these

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 06:30, fjsdfj1284
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2
Do you know the correct answer?
When an electron in a 2p orbital of a particular atom makes a transition to the 2s orbital, a photon...
Questions in other subjects:



Social Studies, 15.07.2019 19:50

Health, 15.07.2019 19:50



History, 15.07.2019 19:50



History, 15.07.2019 19:50