
Chemistry, 18.01.2020 00:31, gabriella1232002
Raising the temperature of 10.0 g of water from 10.0°c to 20.0°c requires 100.0 cal of energy, while raising the temperature of 10.0 g of aluminum from 10.0°c to 20.0°c requires 22 cal. more calories are required to heat the water

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, aedmund1225
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
Do you know the correct answer?
Raising the temperature of 10.0 g of water from 10.0°c to 20.0°c requires 100.0 cal of energy, while...
Questions in other subjects:

Geography, 09.10.2019 07:30


English, 09.10.2019 07:30


Health, 09.10.2019 07:30





Mathematics, 09.10.2019 07:30


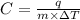
 = change in temperature of substance
= change in temperature of substance



