Chemistry, 30.01.2020 04:02, michaellangley
A10.0 milliliter sample of naoh (aq) is neutralized by 40.0 milliliters of 0.50 m hcl. what is the molarity of the naoh (aq)?
the answer is 2.0m but i am unsure how to do the math. someone

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, toniawu18
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 07:00, jboii11
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2

Chemistry, 23.06.2019 09:00, hunterwilliams375
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
Do you know the correct answer?
A10.0 milliliter sample of naoh (aq) is neutralized by 40.0 milliliters of 0.50 m hcl. what is the m...
Questions in other subjects:

English, 11.10.2019 00:00


Biology, 11.10.2019 00:00





Mathematics, 11.10.2019 00:00

English, 11.10.2019 00:00

Mathematics, 11.10.2019 00:00



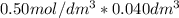
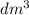
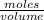
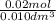
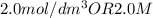
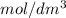 is the same as M
is the same as M