
The partial pressure of co2 gas in an unopened carbonated soft drink is 4.0 atm at 25°c. henry's law constant for co, in the soft drink is 3.1 x 102 mol/l atm at 25°c. the mole fraction of co2 in air at sea level is 3.3 x 104. a. what is the solubility of co2 gas-in-an-unopened soft drink bottle? nopened soft drink bottle? 3.1 x100/l x 4: 0 3 = 0.12 mol/l b. how many moles of co, gas are dissolved in an unopened 2 liter soft drink bottle? c. what is the solubility of co, gas in the opened soft drink at sea level? d. how many moles of co, gas are dissolved in 2 liters of soft drink left open at sea level?

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1

Chemistry, 23.06.2019 01:00, akluke6059
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
Do you know the correct answer?
The partial pressure of co2 gas in an unopened carbonated soft drink is 4.0 atm at 25°c. henry's law...
Questions in other subjects:



Physics, 07.04.2021 16:50




Mathematics, 07.04.2021 16:50

Biology, 07.04.2021 16:50

Mathematics, 07.04.2021 16:50


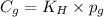
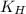 = Henry's constant
= Henry's constant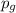 = partial pressure of gas
= partial pressure of gas 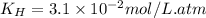
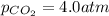
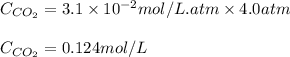
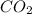 gas-in-an-unopened soft drink bottle.
gas-in-an-unopened soft drink bottle.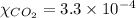
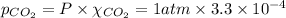
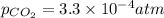
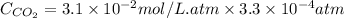
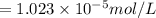
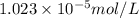 is the solubility of
is the solubility of 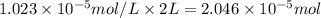
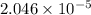 moles of [tex]CO_2[tex] gas are dissolved in 2 liter soft drink left open at sea level?
moles of [tex]CO_2[tex] gas are dissolved in 2 liter soft drink left open at sea level?



