
Suppose we have a 12.2 l sample containing 0.50 mol oxygen gas at a pressure of 1 atm and a temperature of 25 degrees celcius. if all this oxygen were converted to ozone at the same temperature and pressure, what would be the volume of the ozone? balanced equation: 3o2 → 2o3a. 6.5lb. 2.8lc. 8.1ld. 7.4l

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, tntaylor862
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1


Do you know the correct answer?
Suppose we have a 12.2 l sample containing 0.50 mol oxygen gas at a pressure of 1 atm and a temperat...
Questions in other subjects:

History, 06.05.2020 18:05


English, 06.05.2020 18:05



Mathematics, 06.05.2020 18:05

English, 06.05.2020 18:05

History, 06.05.2020 18:05

History, 06.05.2020 18:05


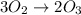
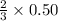 moles of ozone
moles of ozone



