
Chemistry, 16.01.2020 08:31, leslieperez67
The reaction 3 bro- (aq) --> bro3- (aq) + 2 br - (aq) in basic solution is second order in bro-(aq) with a rate constant equal to 0.056 m-1s-1 at 80 degrees celcius. if [bro- ]0 = 0.212 m, then what will [bro- ] be 1.00 min later?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 18:30, tae8002001
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1
Do you know the correct answer?
The reaction 3 bro- (aq) --> bro3- (aq)...
Questions in other subjects:

SAT, 15.03.2022 19:10

Social Studies, 15.03.2022 19:10





Computers and Technology, 15.03.2022 19:10

Social Studies, 15.03.2022 19:10



![r = k[BrO^-]^2](/tpl/images/0457/4932/d05c6.png)
![\dfrac{1}{[BrO^-]_t} = kt + \dfrac{1}{[BrO^-]_o}](/tpl/images/0457/4932/8a81c.png)
 is a rate constant,
is a rate constant,![[BrO^-]_t](/tpl/images/0457/4932/13761.png) is the molarity of the reactant at time t,
is the molarity of the reactant at time t,![[BrO^-]_o](/tpl/images/0457/4932/bdf8a.png) is the initial molarity of the reactant.
is the initial molarity of the reactant.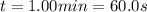
![[BrO^-]_t = \dfrac{1}{kt + \dfrac{1}{[BrO^-]_o}}](/tpl/images/0457/4932/d7afe.png)
![[BrO^-]_t = \dfrac{1}{0.056 M^{-1}s^{-1}\cdot 60.0 s + \dfrac{1}{0.212 M}} = 0.124 M](/tpl/images/0457/4932/c3375.png)





