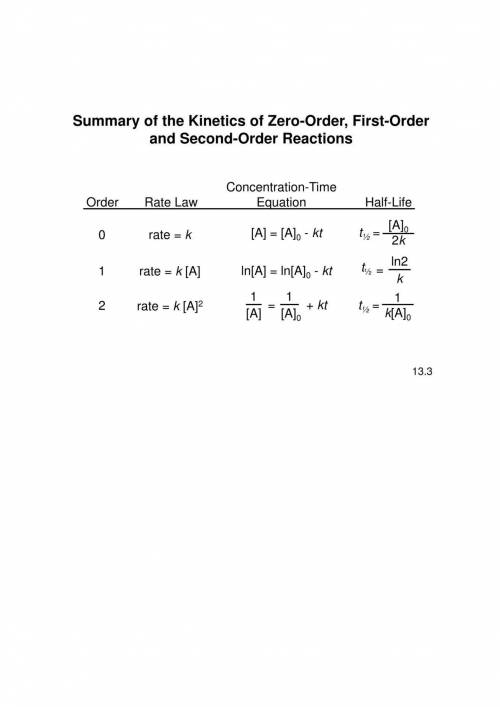
The gas-phase decomposition of ch3cho (g) occurs according to the equation
ch3cho (g) > ch...

The gas-phase decomposition of ch3cho (g) occurs according to the equation
ch3cho (g) > ch4 (g) +co (g) and is second order. the value of the rate constant is 0.105 m-1 x s-1 at 490 degrees celcius. if the concentration of ch3cho (g) is 0.012 m initially, what will be its concentration 5.0 minutes later.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, itzhari101
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1
Do you know the correct answer?
Questions in other subjects:











![[A] =8.71\times 10^{-3}](/tpl/images/0457/3112/8b81b.png) M or 0.00871 M
M or 0.00871 M![\frac{1}{[A]}=\frac{1}{[A_{0}]}+kt](/tpl/images/0457/3112/b721a.png)
![[A_{0}]](/tpl/images/0457/3112/747e3.png) = Initial concentration
= Initial concentration![\frac{1}{[A]}=\frac{1}{0.012}+ 0.105\times 300](/tpl/images/0457/3112/e46ed.png)
![\frac{1}{[A]}=83.33+ 31.5](/tpl/images/0457/3112/c3da5.png)
![\frac{1}{[A]}=114.83](/tpl/images/0457/3112/63efb.png)
![[A] =\frac{1}{114.83}](/tpl/images/0457/3112/33594.png)