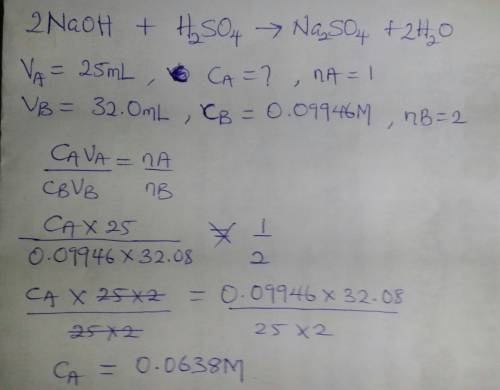Chemistry, 16.01.2020 04:31, risolatziyovudd
Asolution of sulfuric acid is titrated with a solution of sodium hydroxide to the phenolphthalein end point. the sample of sulfuric acid is 25.00 ml. the titration takes 32.08 ml of 0.09946 m sodium hydroxide.
a.) write out a balanced equation for the reaction.
b.) calculate the molarity of the sulfuric acid.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1


Chemistry, 22.06.2019 22:30, SavageKidKobe
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 06:20, raidattarab
What is the magnitude of the force of gravity between to 1000 kg cars which are separated by distance of 25. 0 km on an interstate highway? the force between the two cars will be what
Answers: 3
Do you know the correct answer?
Asolution of sulfuric acid is titrated with a solution of sodium hydroxide to the phenolphthalein en...
Questions in other subjects:





Biology, 26.08.2021 20:30


Mathematics, 26.08.2021 20:30

Mathematics, 26.08.2021 20:30

Biology, 26.08.2021 20:30

English, 26.08.2021 20:30