
Chemistry, 15.01.2020 23:31, kookycookiefanx
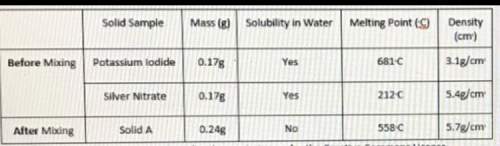
On lab day. milania did an experiment with two solids: potassium iodide and silver nitrate. milania measured and recorded the mass, solubility, melting point, and calculated
the density of potassium iodide and silver nitrate before mixing them together. she then calculated the same properties of solid a which resulted after she mixed
potassium iodide and silver nitrate. her experimental results are shown below
a. it is a physical change because mass of solid a is similar but o the masses of potassium iodide and silver nitrate
b. it is a physical change because the solubility of solid a is different than potassium iodide and silver nitrate
c. it is a chemical change because the densities of potassium iodide and silver nitrate are the same
d. it is a chemical change because solubility in water of solid a is different than the solubility in water or both potassium iodide and silver nitrate

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mommatann
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 21.06.2019 20:30, dwighthibbert56
What was the procedure by which case united states vs lopez went to court
Answers: 1


Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2
Do you know the correct answer?
On lab day. milania did an experiment with two solids: potassium iodide and silver nitrate. milania...
Questions in other subjects:


Biology, 20.01.2022 14:40


English, 20.01.2022 14:40

Chemistry, 20.01.2022 14:40


Health, 20.01.2022 14:50

Chemistry, 20.01.2022 14:50


Mathematics, 20.01.2022 14:50






