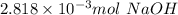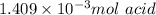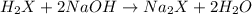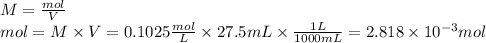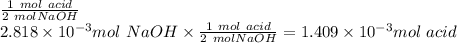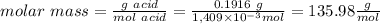Chemistry, 15.01.2020 19:31, eyeneedalife
1) a student dissolved 0.1916 g of an unknown diprotic acid in 100 ml of distilled water. the acid was then titrated with 0.1025m naoh solution. the second equivalence point showed the sharpest change in ph, and so it was used to determine the molar mass of the unknown acid. the volume of naoh needed to reach the equivalence point was 27.5 ml.
a. calculate the number of moles of naoh used in the titration to reach the second equivalence point.
b. calculate the number of moles of diprotic acid, based on the fact that we are examining the second equivalence point.
c. calculate the molar mass of the diprotic acid.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2



Chemistry, 22.06.2019 23:00, lulprettyb
What is the most common reason for matter changing its state?
Answers: 1
Do you know the correct answer?
1) a student dissolved 0.1916 g of an unknown diprotic acid in 100 ml of distilled water. the acid w...
Questions in other subjects:

Computers and Technology, 02.10.2019 11:50

Physics, 02.10.2019 11:50




Biology, 02.10.2019 11:50

Mathematics, 02.10.2019 11:50

English, 02.10.2019 11:50