Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2


Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 02:00, sakria2002
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
Do you know the correct answer?
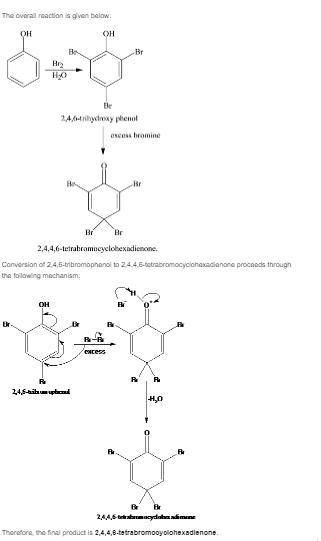
Treatment of phenol with excess aqueous bromine is actually more complicated than expected. a white...
Questions in other subjects:


Mathematics, 15.10.2021 01:00

Social Studies, 15.10.2021 01:00

World Languages, 15.10.2021 01:00

Mathematics, 15.10.2021 01:00




Mathematics, 15.10.2021 01:00

Biology, 15.10.2021 01:00