Chemistry, 15.01.2020 06:31, santiagobermeo32
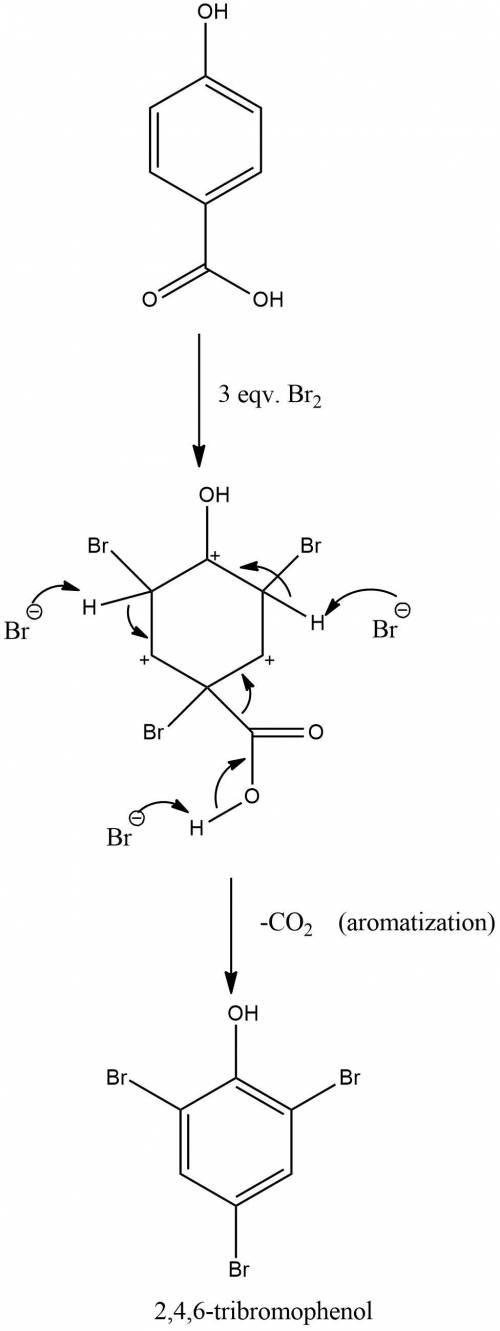
Treatment of p-hydroxybenzoic acid with aqueous bromine leads to the evolution of carbon dioxide and the formation of 2,4,6-tribromophenol. explain.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, sammiehammer
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2


Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3
Do you know the correct answer?
Treatment of p-hydroxybenzoic acid with aqueous bromine leads to the evolution of carbon dioxide and...
Questions in other subjects:

Law, 21.12.2020 06:00



Physics, 21.12.2020 06:00


History, 21.12.2020 06:00

Mathematics, 21.12.2020 06:00

Physics, 21.12.2020 06:00


English, 21.12.2020 06:00